Bladder cancer
(Non-Muscle Invasive Bladder Cancer)
Problem: lack of effective bladder cancer therapeutics, in particular for early-stage bladder cancer
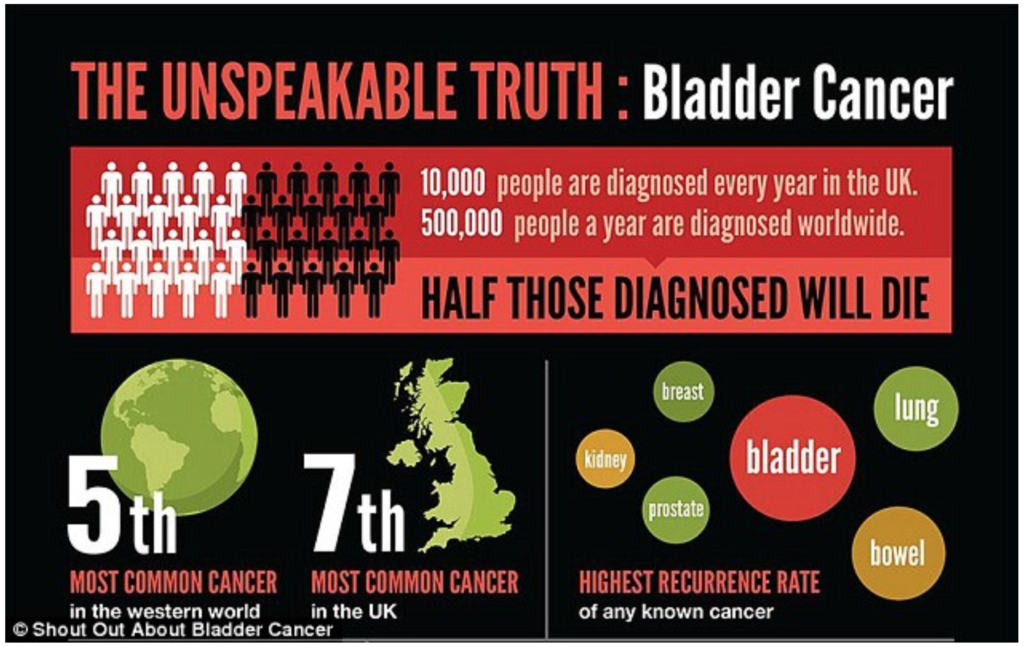
Bladder cancer is the fifth most common cancer in the European Union (EU) and has the highest recurrence rate of all cancer indications. Each year, around 500,000 patients worldwide are diagnosed with bladder cancer, and this number is rising. Worldwide, bladder cancer results in around 165,000 deaths each year. The high mortality of bladder cancer is mainly caused by the lack of effective therapies and the high recurrence rate (~70%). Moreover, bladder cancer places a significant burden on the health care system with high healthcare expenses. In fact, it accounts for the highest treatment cost per patient in the cancer area. The total costs associated with bladder cancer treatment in the EU amount to €4.9 billion (2012), of which €2.9 billion are direct treatment costs. These data clearly demonstrate the significance of bladder cancer as a health care problem.
Bladder cancer is classified into three different clinical stages:
- Non-muscle invasive bladder cancer (NMIBC): Stages Ta, T1 and Tis/CIS
- Locally invasive bladder cancer: Stages T2 and T3
- Metastatic bladder cancer (cancer spreading to other parts of the body): Stage T4
At diagnosis, around 70% of the diagnosed bladder cancers are classified as NMIBC, corresponding to 1.6 million patients currently suffering from NMIBC in the EU. The main challenge in NMIBC management is to prevent progression towards metastatic bladder cancer. At present, however, all available treatment options for NMIBC are suboptimal. They lack tumor specificity, are not effective, and cause significant side-effects. To prevent progression towards muscle invasion, and thus metastatic bladder cancer, radical removal of the bladder (‘cystectomy’) is often the only treatment option, which is a drastic surgical procedure, significantly impairing the patient’s quality of life. In view of the high risk of tumor recurrence and the limited treatment options, there is an urgent need for novel NMIBC treatments that combine efficacy and selectivity for the targeted tumor, without causing side-effects.