Infections continue to threaten human health. Pathogenic bacteria rapidly develop resistance against antibiotics. Antibiotic resistance is a global, rapidly growing health concern. The World Health Organization (WHO) has declared antibiotic resistance a major threat to global health. Hamlet Biopharma develops novel therapies against infection with the goal to provide a immunotherapy as a molecular framework for the treatment of bacterial infections; much needed to combat antibiotic resistance. One of the main drug candidates, the Interleukin-1 receptor antagonist (IL1-RA), has successfully been used in the clinic to treat bladder pain syndrome and is currently being investigated in controlled Phase II trials.
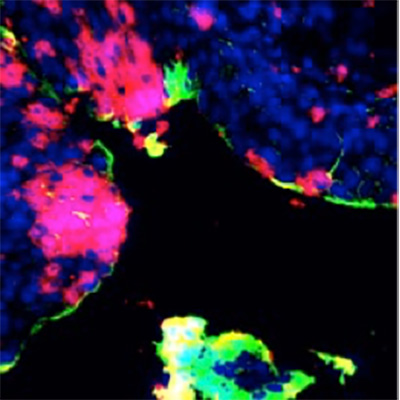
IL1-receptor antagonists
IL-1 receptor antagonist (Kineret) to treat acute infections and prevent tissue damage
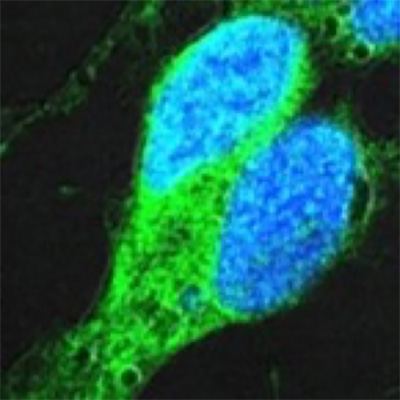
NK1R-inhibitors
A nerve cell receptor antagonist prevents pain and tissue damage during acute infections
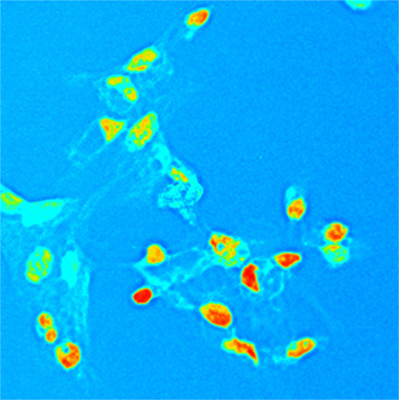
RNA Pol II inhibitors
A small molecule from ”nice bacteria” prevents over-activation of immunity and reduces inflammation
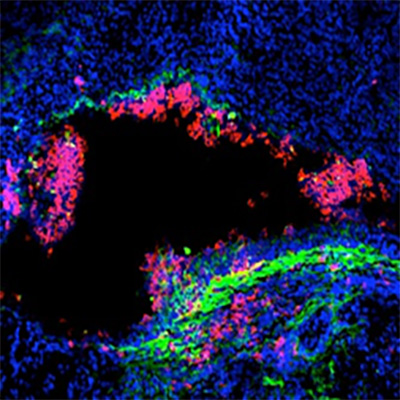
IRF7 inhibitors
New molecular tools to inhibit ‘’bad’’ inflammation in infected tissue and reduce the risk of sepsis
Immunomodulation offers a new strategy to treat bacterial infections
Hamlet BioPharma is driving the development of four projects with drug candidates in different stages of development as shown below.
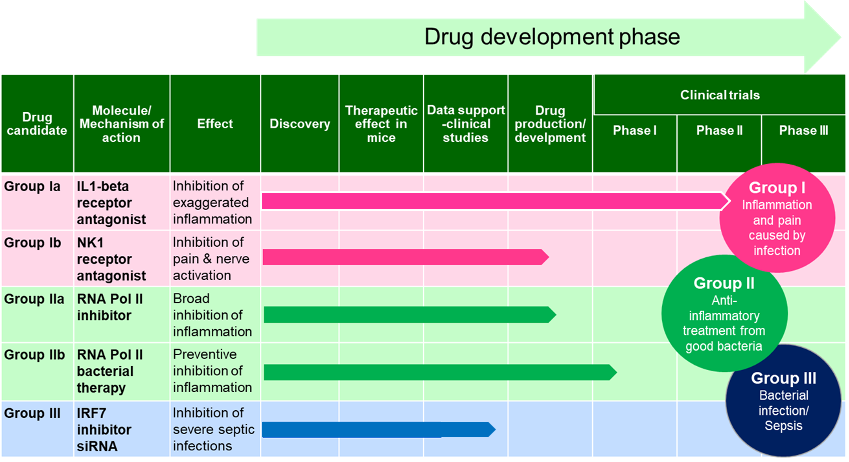
Drug candidates in different stages of development

Clinical studies (Phase II) of IL-1RA for two indications
1. IL-1RA – Bladder Pain Syndrome
A mechanism for immune system hyperactivation in acute cystitis has been identified and the disease has been successfully treated by inhibiting this mechanism. A first study with IL-1 receptor antagonist has been conducted in patients with chronic bladder pain syndrome, with long-term follow-up in 2021. A clear and lasting positive effect was demonstrated. The results provide a basis for conducting a controlled clinical study in this indication.
Bladder pain syndrome is a chronic condition that involves severe pain and has a major negative impact on the quality of life of those affected. Several treatment methods have been tested, but currently there are no specific treatments available for those affected. Neither ordinary painkillers nor even morphine provide sufficient relief. The pain often recurs, even with surgical removal of the damaged parts of the bladder.

Hamlet BioPharma has previously reported positive treatment results in this patient group with IL-1α and β receptor antagonist (IL-1RA) anakinra. The results show relief of symptoms and increased quality of life. The study also showed positive long-term effects in the patients who continued their treatment.
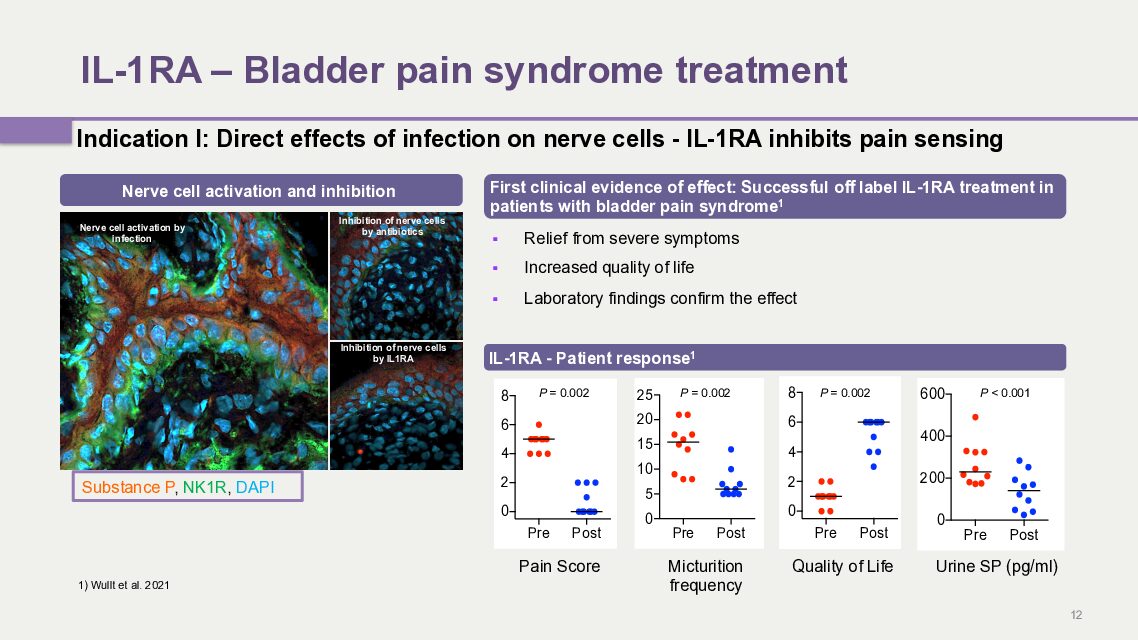
Hamlet BioPharma is now continuing with a randomized, placebo-controlled trial of IL-1RA in patients with bladder pain syndrome. The company has received permission from the Swedish Medical Products Agency to conduct the study, which is planned to include several different clinical centers.
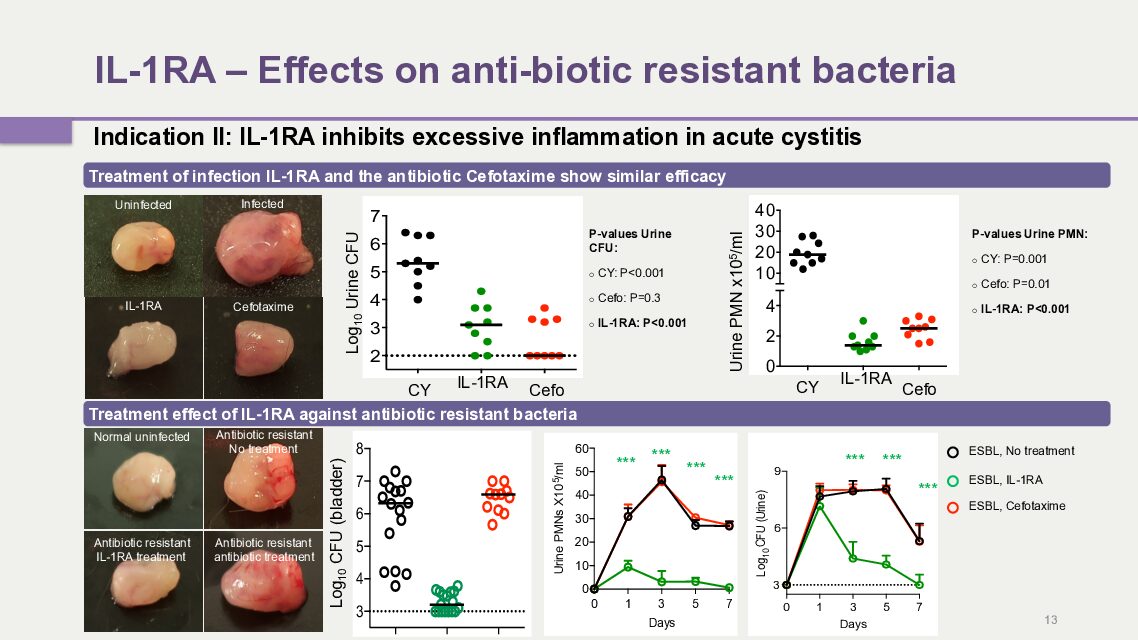
2. IL-1RA –Recurrent Acute Cystitis
In August 2021, a clinical Phase II study for acute recurrent cystitis was initiated in Giessen, Germany. The majority of patients have been included and the study is progressing according to plan. Hamlet BioPharma announced strong positive results from the study in a press release September 26th, 2024.
The Phase II study compared the efficiency of immunotherapy to antibiotics in patients with recurrent acute cystitis, a common and debilitating infection. This study is the first to introduce immunotherapy as a novel treatment option in patients with recurrent infections, where antibiotics are the standard of care. The results suggest that the majority of patients respond to either treatment, with a reduction of symptoms and an improved quality of life, suggesting a great potential for immunotherapy as a complement or substitute for antibiotics.
Key findings from the phase II study
- The study included patients with severe, recurrent urinary tract infections and compared antibiotic treatment to immunotherapy.
- Treatment was randomized and patients received either antibiotic treatment or treatment with anakinra, an Interleukin-1 receptor antagonist (IL-1RA).
- The results showed that patients responded positively to both treatments, with a significant reduction in symptoms, short term and long term, as well as fewer symptomatic relapses and an improved quality of life.
- The analysis shows no difference in effect between Immunotherapy and antibiotic treatment, which is the most favourable outcome.
- The results open the door to introducing immunotherapy with IL-1RA for repeated infections in this large patient group, thereby reducing the need for antibiotic treatment.
- The results are also important for the normal bacterial flora, which is disturbed by repeated antibiotic treatment.
- The reduction of antibiotic use in this large patient group may also contribute to the fight against antimicrobial resistance.
In a recent study in mice, IL-1RA treatment also accelerated bacterial clearance from infected bladders and kidneys, including antibiotic resistant bacteria, where antibiotic treatment was inefficient.

Protective molecules from friendly bacteria improve the balance of the immune system.
Pre-clinical treatment efficacy against infection
The aim is to understand how proteins from ”friendly” bacteria can be useful and to develop discoveries in this area further for future infection treatment.
A protein with positive effects, NlpD, has been successfully used as a therapy for acute cystitis in an animal model.
The bacterial protein will be manufactured on a larger scale, tested for toxicity and efficacy, and further developed towards clinical trials. A manufacturing collaboration with a world-leading producer has been initiated.
The proteins from ”friendly” bacteria also have the potential to suppress general inflammatory states. These effects shall be investigated in animal models for relevant inflammatory diseases.
Inhibitors of IRF7 – siRNA technology protects against renal inflammation and urosepsis in an animal model.
Pre-clinical treatment efficacy against infection
Renal pelvic inflammatory disease is the most serious form of urinary tract infection and is caused by bacteria that infect the kidneys themselves. In about 30% of adults who get acute renal pelvic inflammatory disease, the bacteria also invade the bloodstream, causing urosepsis and without appropriate treatment, mortality is high. Acute renal pelvic inflammatory disease is also common in children and can cause long-term problems by damaging the kidney tissue.
The company intends to drive the development of this siRNA-based treatment of severe bacterial infections in the kidneys that have spread to the blood. The condition urosepsis is life-threatening and a common cause of death in elderly care. The company will seek collaborations to develop this technology towards clinical practice.
Read publications
-
Immunomodulation – a molecular solution to treating patients with severe bladder pain syndrome? Wullt, B. et al. European Urology Open Science, 31, 49-58; doi:10.1016/j.euros. 2021.07.003 (2021).
-
Active bacterial modification of the host environment through RNA Polymerase II inhibition. Ambite, I. et al. The Journal of Clinical Investigation131(4), e140333, doi:10.1172/JCI140333 (2021).
-
Immunomodulation therapy offers new molecular strategies to treat UTI. Butler, D. et al. Review Article, Nature Reviews Urology, 19, 419-437; doi:10.1038/s41585-022-00602-4 (2022).
-
Therapeutic Effects of IL-1RA against Acute Bacterial Infections, including Antibiotic-Resistant Strains. Ambite, I. et al. Pathogens, 13(1), 42; doi:10.3390/pathogens13010042, (2024).
