Mechanism of Action
Alpha1H and the predecessor molecule, HAMLET, are tumoricidal protein-lipid complexes with broad effects against cancer cells of different origins.
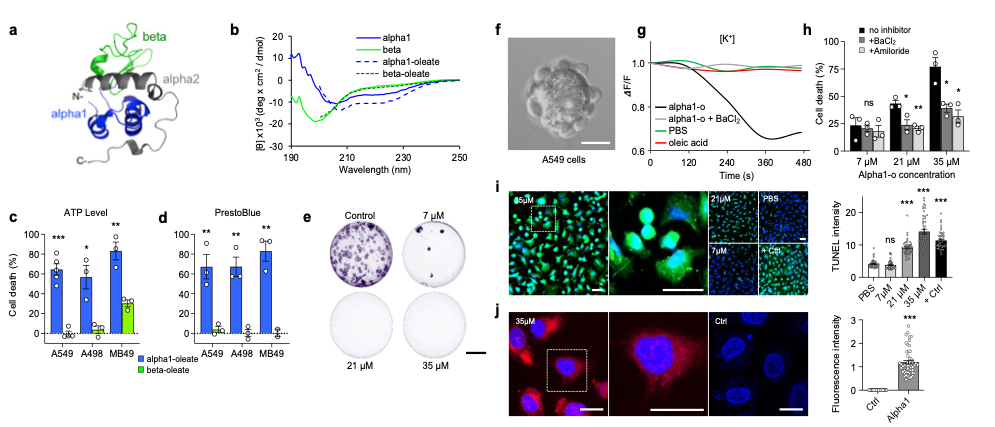
The involvement of specific alpha-lactalbumin peptide motifs in tumor cell death, was examined by synthesizing the N-terminal alpha-helical domain (residues 1-39) or the beta sheet (residues 40-80) domains of human α-lactalbumin (Nadeem et al., 2019, Brisuda et al., 2021). The alpha1 peptide formed complexes with oleic acid (Alpha1H, 1:5) and circular dichroism (CD) spectra detected an increase in alpha-helical structure content in the Alpha1H complex.
Alpha1H triggered a rapid, dose-dependent cell death response, quantified by ATP levels and PrestoBlue fluorescence in human lung (A549), kidney (A498) and murine bladder (MB49) cancer cells. In comparison, the beta-oleate complex lacked tumoricidal activity and tumor cells incubated with individual alpha-helical peptides (35 μM) or oleic acid (175 μM) were not killed. The loss of cell viability was irreversible, as shown after 10 days, using colony assays. It was concluded that the alpha1 peptide forms a tumoricidal complex with oleic acid, which is functionally similar to HAMLET (Brisuda et al., 2021).
HAMLET and Alpha1H initiate the cell death response by integration into the plasma membranes of tumor cells (Storm et al., 2013, Nadeem et al., 2015, Brisuda et al., 2021). The membrane lipid composition differs between healthy differentiated cells and tumor cells and results indicate that membrane lipid conformations serve as surrogate receptors for subsequent signal transduction, leading to tumor cell death (Nadeem et al., 2015, Ho et al., 2022). Cell membrane responses were observed via rapid Na+ and K+ fluxes and pretreatment of the cells with Na+ or K+ flux inhibitors reduced cell death by 40-50%, linking the membrane response and ion flux to tumor cell death (Figure 1).
Finally, the Alpha1H complex (labeled with AlexaFluor568) is rapidly internalized by A549 tumor cells and translocates to the nuclei, where it triggers double-strand DNA breaks as measured by TUNEL staining. The cells die by an apoptosis-like mechanism (Figure 1).