Tuberculosis
Tuberculosis is one of the top ten causes of death worldwide and the most common cause from a single infectious agent. Current treatment protocols require a cocktail of antibiotics given by skilled medical staff over six months. There is a growing problem of drug resistance, which reduces the options for this patient group.
Hamlet Biopharma will develop a new therapeutic concept to fight tuberculosis using bactericidal peptide drug candidates that show treatment efficacy in animal models.
Tuberculosis (TB) is caused by Mycobacterium tuberculosis and the infections mostly start in the lungs. TB is one of the main causes of death worldwide and about a quarter of the world’s population has latent TB, and are carriers but not clinically ill.
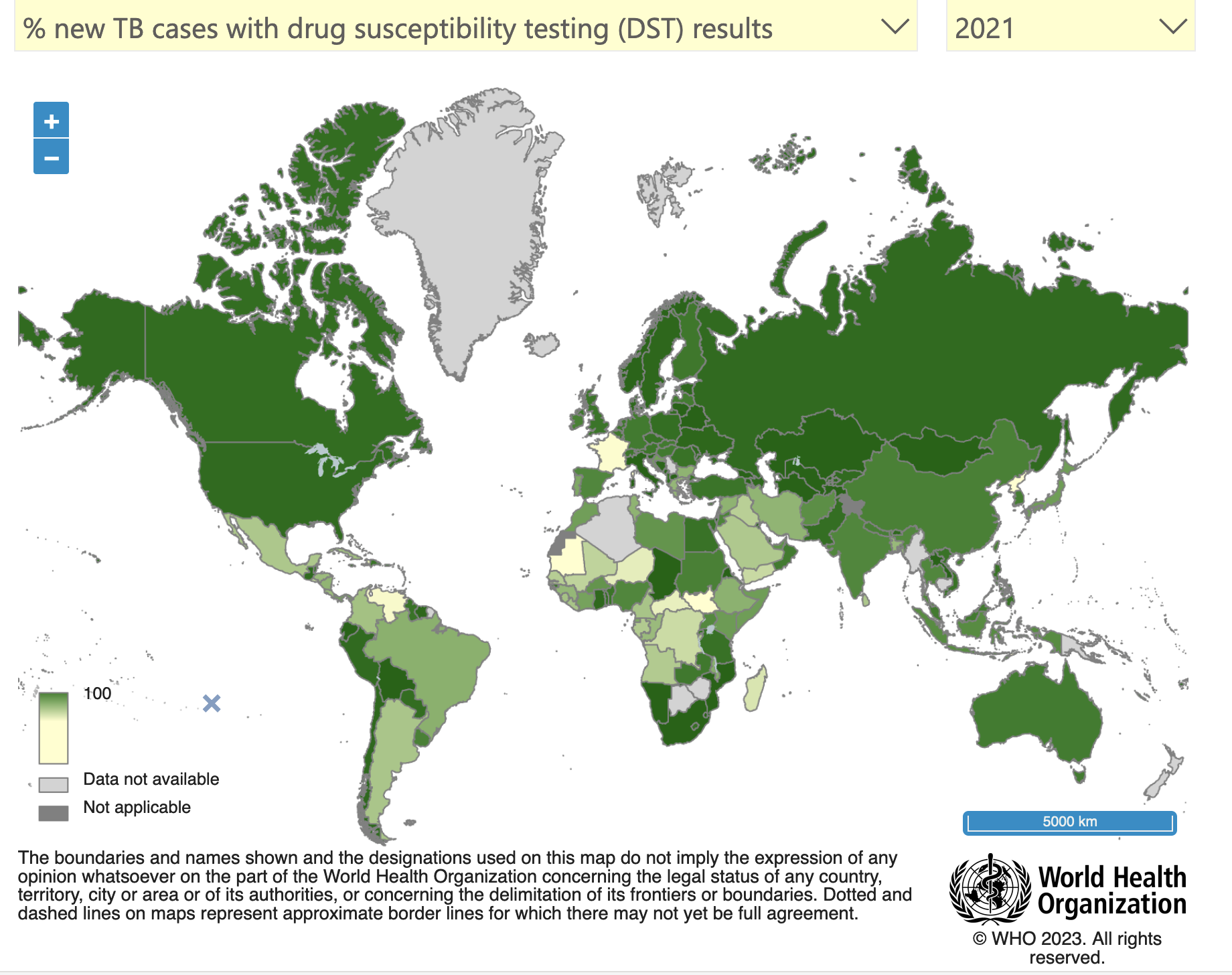
In 2021, approximately 10.6 million people fell ill with TB, and 1.6 million died from the disease (including 0.2 million individuals with HIV). Multidrug-resistant TB (MDR-TB) remains a health security threat and the lack of efficient treatments has created public health crisis. The WHO estimates that last year there were 558 000 new cases with resistance to rifampicin – the most effective first-line drug, of which 82% had MDR-TB.
Treatment for drug-resistant TB has increased the number of drugs used and the treatment time. The new peptide drug works also against infections caused by resistant MDR-TB.
Antimicrobial peptides
Originally the peptide was isolated from the fungus Pseudoplectania nigrella, also called “ebony cup”. The peptide is active against clinical isolates of multi-drug resistant tuberculosis bacteria in the test tube and has shown potent therapeutic effects in animal models of tuberculosis.
The peptide has been found not to be toxic and is resistant to degradation. There is no evidence of resistance development. The peptide remains stable after intravenous administration in several murine TB infection models. In murine TB models the peptide eliminated M. tuberculosis with efficiency comparable to Rifampicin, which frequently is used to treat tuberculosis and showed an additive effect with isoniazid and ethambutol.
This peptide will now be produced in larger quantities, subjected to further toxicity studies and prepared for clinical trials.
