Newsletter
SUCCESSFUL PHASE II STUDIES FOR THREE CLINICAL INDICATIONS
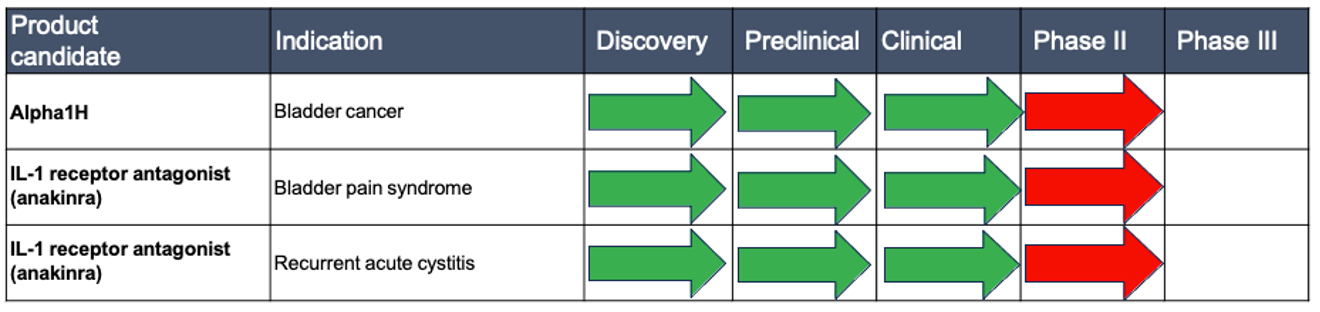
Successful Phase II studies in cancer and infections.
Hamlet BioPharma is the pharmaceutical company with a strong portfolio of projects for the treatment of cancer and infections. Hamlet BioPharma has now completed Phase II clinical trials and successfully treated patients with three different diseases.
- Treatment of bladder cancer patients with Alpha1H reduces the number and size of tumors.
- Treatment of patients with recurrent urinary tract infections with the IL-1 inhibitor anakinra reduces pain and recurrence, with as good an effect as antibiotics.
- Treating patients with severe bladder pain with anakinra reduces symptoms, improves quality of life and has long-term benefits.
CANCER
NEW CLASS OF ANTI-CANCER DRUGS
Hamlet BioPharma’s goal is to develop drugs that specifically target tumor cells without harming healthy tissue, with reduced risk of side effects for the patient. The HAMLET family of drug candidates effectively kills cancer cells and growing tumor tissue with high precision. In animal models of bladder cancer, colon cancer and brain tumors, HAMLET has shown good treatment effects without damage to healthy tissue. The development also aims to produce drugs for the treatment and prevention of other cancers.
BLADDER CANCER
Bladder cancer is a common form of cancer that is difficult to treat, problematic for the patient and costly for society. When treatment is not optimal, the recurrence rate is high, up to 70-80% after the first surgical treatment. Early bladder cancer has the highest recurrence rate and treatment cost per patient among all cancer types (total cost in Europe: >€4.9 billion). Bladder cancer is also one of the most commonly diagnosed cancers worldwide. According to the latest statistics from the World Cancer Research Fund International, over 614,298 cases of bladder cancer were diagnosed globally in 2022, with a significant proportion being superficial bladder cancer (WCRF International). This cancer is treated with surgery to remove the tumor, followed by chemotherapy or other treatments, depending on the severity of the tumor as determined by the tissue analysis performed during surgery. Around 15% of patients with an initial superficial bladder cancer later develop a more severe, muscle-invasive form that can metastasize (Babjuk, Cancer Central).
ALPHA1H TREATMENT OF BLADDER CANCER
Hamlet BioPharma chose early bladder cancer as the first indication based on studies with HAMLET, which have shown clear effects on this particular type of cancer. There is a great need for new, more effective and safe treatment options for this large and growing patient group. Alpha1H has powerful positive effects in animals with bladder cancer and the clinical study programme has been designed with high precision thanks to the extensive research. In preparation for the clinical trials, Alpha1H has been analyzed for toxicity in detail by external experts and no toxicity has been detected when treating healthy animals.
Part 1 - A randomized, placebo-controlled trial
A randomized, placebo-controlled study of Alpha1H was carried out by a team of doctors at a Hospital in Prague in collaboration with Hamlet BioPharma with good results. Patients did not experience any severe side effects from Alpha1H. The treatment had clear effects on the tumor, which decreased significantly in size.
Cells and pieces of the tumors were detached from the tumor, and analysis of urine samples showed large amounts of cells that had taken up the medicine. The tumor also showed evidence of uptake of Alpha1H and apoptosis, which is a beneficial form of cell death. Advanced molecular analyses also showed interesting and specific responses in tumors treated with Alpha1H, for example that Alpha1H switching off the expression of a large number of different cancer genes, which proved to be an important milestone.
The figure gives an overview of the placebo-controlled study and treatment of bladder cancer with Alpha1H. The images show how Alpha1H is administered and how different effects can be evaluated using clinical techniques and in different patient samples. Patients secrete large amounts of cells, often containing the drug, the tumor shrinks in size and analysis of immune responses provides important information on the effect on tumor tissue.
Successful placebo-controlled study published in Nature Communications >>

Part-2 - Increasing clinical efficacy with higher doses of Alpha1H
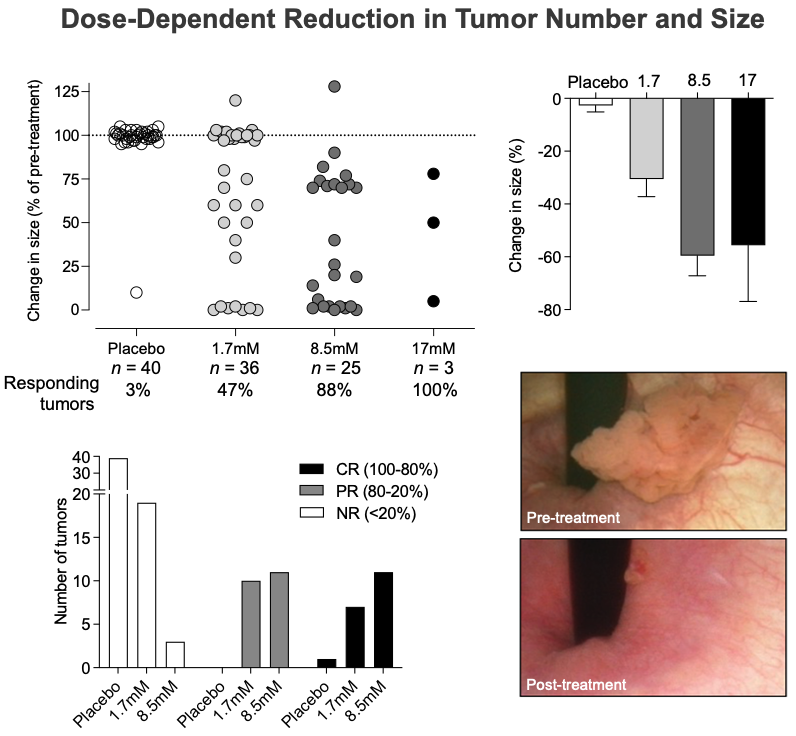
In preparation for Phase III trials, it is important to determine the dose of the drug that provides optimal patient safety and treatment efficacy. In animal models, the effect of Alpha1H against bladder cancer has been shown to increase dramatically with increasing dose without causing side effects.
Results of the combined analysis of the two clinical parts are summarized below:
- Reduction in tumor size
-
- Treatment resulted in a complete or partial response in 88% of tumors treated with 8.5 mM and in 47% treated with 1.7 mM Alpha1H.
- Changes in the tumor
-
- The treatment resulted in the dissolution of the tumor and the release of fragments and cells into the urine. This effect increased significantly with higher doses of Alpha1H.
-
- The tumor cells died by a mechanism called apoptosis after taking up Alpha1H.
-
- Pieces of tissue from the tumor that remained in the patient after the treatment had lost some of their tumor characteristics and become more like healthy tissue, as shown by gene expression analysis.
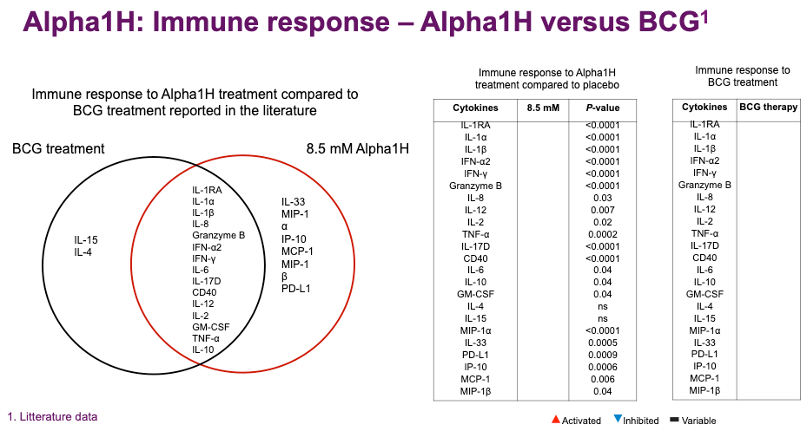
Broad immune response with strong anti-tumor potential in bladder cancer patients
The analyses of the clinical samples have provided important molecular insights that have improved our understanding of the mechanisms of action of Alpha1H and the response of the tumors to treatment. Alpha1H is taken up by the tumors and the cells secreted from the patient’s tumor contain large amounts of Alpha1H. This is significant as it proves that the substance reaches the tumor and has direct effects on the tumor cells within hours of treatment.
The activation of the immune system is another important mechanism of action that explains the potential of Alpha1H. Through the immune system, the tumor can be attacked and neutralized, as Alpha1H creates a multifunctional therapeutic environment in the tissue.
The manuscript describing this study has been published in Cancer Medicine >>
Part 3 - Extended treatment with Alpha1H
Hamlet BioPharma has completed the third part of the clinical trial in Prague with Alpha1H treatment of patients with bladder cancer. The evaluation of the study is ongoing. The aim of this part of the study is to optimize the tumor treatment.
IMMUNOTHERAPY
IMMUNOTHERAPY – A NEW WAY TO TREAT BACTERIAL INFECTIONS
Hamlet BioPharma also focuses on developing new drugs for treatment of infections and inflammation. The company is developing immunotherapy for bacterial infections as an alternative to antibiotics. Positive clinical effects have been reported in patients with bladder pain and in patients with recurrent acute cystitis.
Antibiotic resistance is one of the biggest problems of our time and a fateful issue for the future. Common bacterial infections, previously treatable, now pose a major threat. Mortality rates increase significantly for severe bacterial infections such as sepsis, pneumonia or kidney infections. As a result, patients with urinary tract infections cannot know whether antibiotics will work or not, and completely different solutions are needed.

Researchers at Lund University, in collaboration with Hamlet BioPharma, have long experience in research on infections and immunity and have identified key control mechanisms for the balance between defense and disease. These molecular insights make it possible to select compounds that are suitable for controlling the immune response and reducing disease caused by infections. One example is the study of the immune molecule Interleukin-1 (IL-1) and its receptor IL-1RA. An existing drug, Kineret, contains the substance anakinra and inhibits the effects of IL-1.
RECURRENT ACUTE CYSTITIS
Recurrent acute cystitis, or recurrent urinary tract infection (UTI), is a very common disease in women. Approximately 60% of women will experience a symptomatic UTI in their lifetime. Of these, 20-40% will experience a further episode of cystitis, and between 25-50% will experience multiple recurrent episodes (AUA Website). The disease can be very distressing and lead to a significant impact on quality of life, especially for women who experience frequent recurrent infections. The treatment and management of recurrent UTIs, especially with increasing antibiotic resistance, is a global challenge that also generates high healthcare costs. Recurrent UTIs are usually treated with short courses of antibiotics, and some patients may also receive preventive treatment or other non-antibiotic strategies to reduce the risk of future infections.
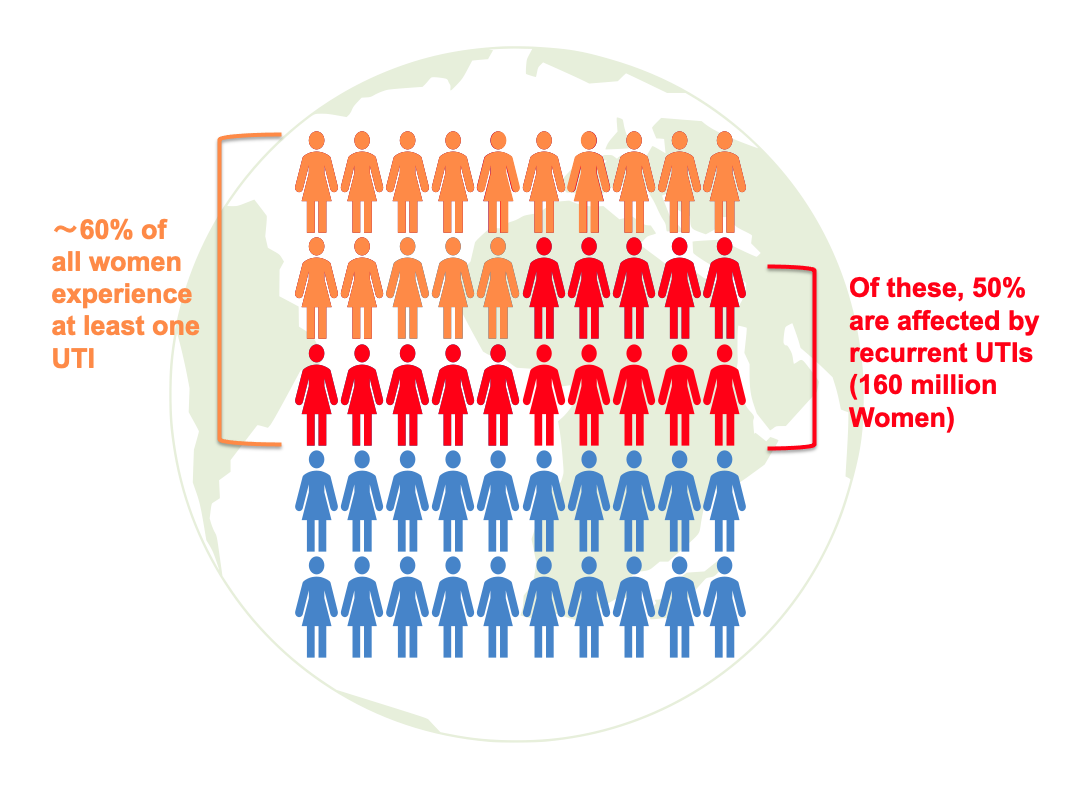
The clinical results support the use of immunotherapy for recurrent infections, representing a paradigm shift
The company has announced positive results from the controlled Phase II clinical trial in patients with recurrent acute cystitis. The study compared antibiotic treatment with immunotherapy (anakinra) in patients with recurrent urinary tract infections, a study conducted in collaboration with specialists in Giessen, Germany. Both treatments showed significant reduction of symptoms and improved quality of life, with no difference in efficacy between the two patient groups.
Immunotherapy with anakinra can thus reduce the need for antibiotics, which is an important aspect in the fight against resistant bacteria. In addition, the normal bacterial flora can be preserved, which in turn helps to counteract antimicrobial resistance. The next step is a more comprehensive analysis of the study results, which will be submitted for scientific publication.
This approach opens up new possibilities to treat antibiotic-resistant bacteria and shifts the focus from direct elimination of bacteria to strengthening the host’s antibacterial defences, offering an alternative solution to the growing global health threat of antibiotic resistance identified by the WHO.
BLADDER PAIN SYNDROME, BPS - A SYNDROME OF SEVERE PAIN IN THE BLADDER
Bladder Pain Syndrome (BPS), also known as interstitial cystitis, is a chronic disease involving bladder pain and discomfort estimated to affect between 4 to 12 million people in the United States, with the majority of cases being women (approximately 80-90%) (ICHelp)(BladderSmart). BPS causes chronic pain and discomfort in the bladder, often without an infection or other identifiable cause. The disease significantly affects quality of life and requires long-term management and treatment.
Patients with BPS have severe pain and other symptoms, which are socially disabling. As conventional painkillers are not effective, some patients are treated with morphine or surgery, often without lasting effects. The compound anakinra (IL-1RA), patented by the company for the treatment of bladder pain conditions, has shown promising effects in patients receiving off-label treatment. Pain scores decreased after treatment and quality of life increased in this severely disabled patient group. In addition, the laboratory tests showed a convincing reduction in pain molecules after treatment, suggesting a direct effect of the treatment at the molecular level.
Hamlet BioPharma is therefore continuing the clinical programme with a controlled phase II study in this patient group. The study was initiated with a screening phase, to identify patients who responded to treatment. A significant proportion of patients treated with anakinra responded favorably to treatment.
MARKET OVERVIEW
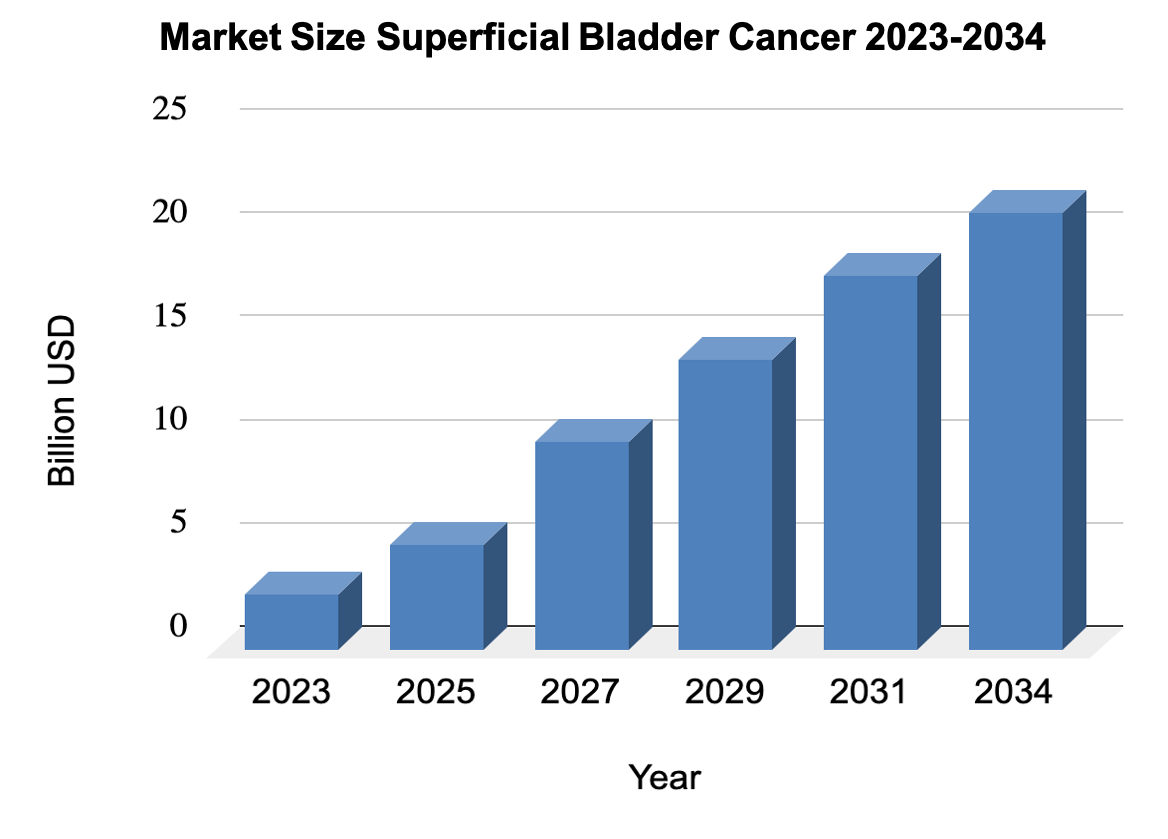
Source: https://www.transparencymarketresearch.com/ non-muscle-invasive-bladder-cancer-market.html
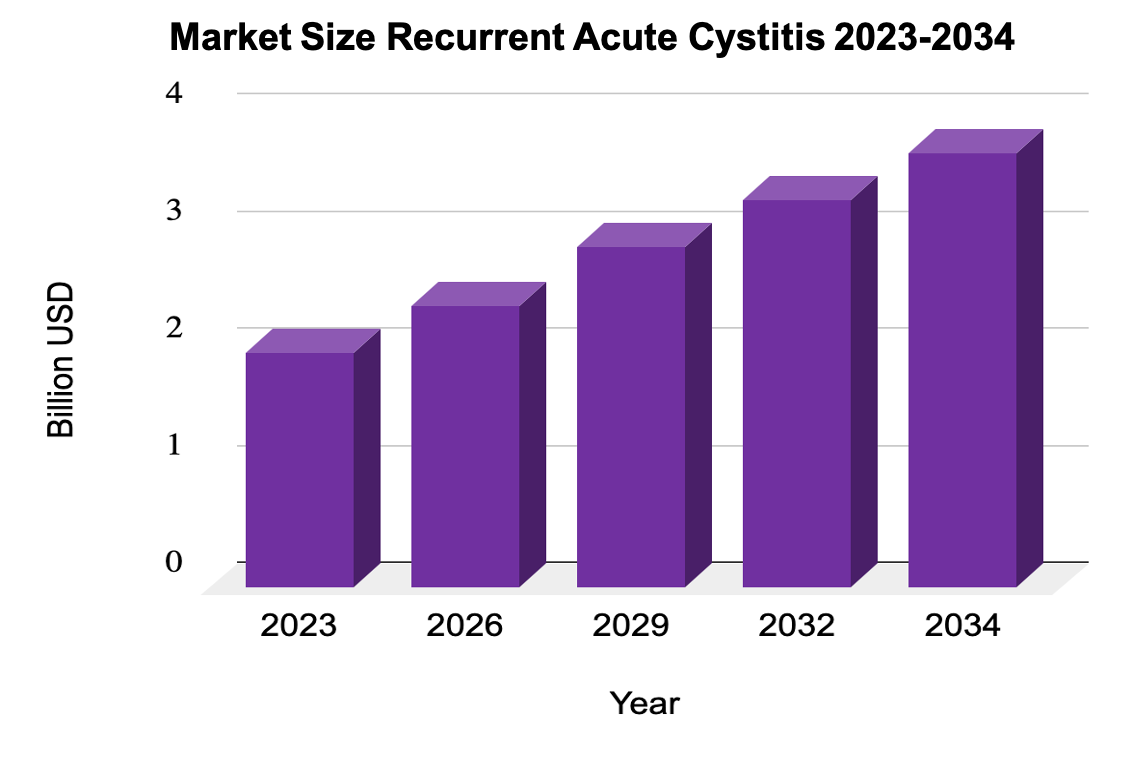
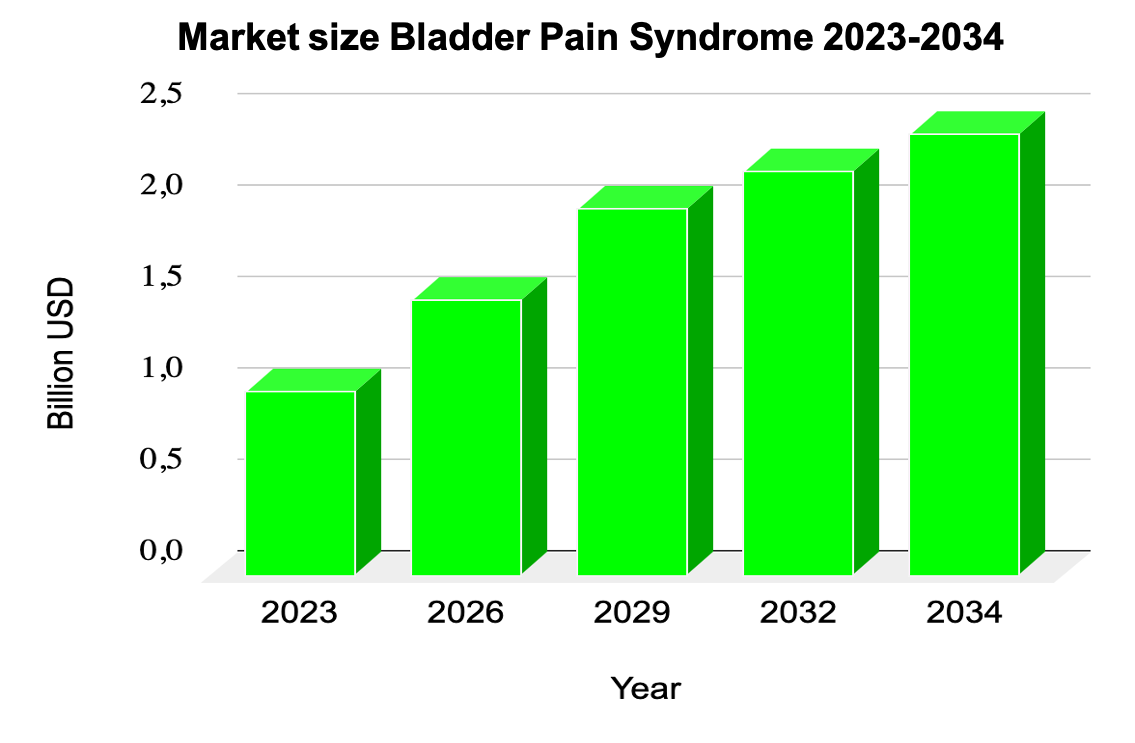
Source: IMARC; https://www.imarcgroup.com/bladder-pain-syndrome-market
PIPELINE
Hamlet BioPharma – the pharmaceutical company with a strong portfolio of projects for the treatment of cancer and infections